
Smog is air pollution that reduces visibility. The term ‘smog’ was first used in 1905 by H.A. Des Voeux to describe a blend of ‘fog’ and ‘smoke’ found over many British towns. Smog can be of two types: classical or sulfurous smog and photochemical smog or industrial smog. The ‘Great Smog of 1952’ in London is an example of sulfurous smog, also called ‘London Smog.’ This article deals with photochemical smog – components, formation, effects, and prevention.

Photochemical smog, also known as ‘summer smog,’ is formed when ultraviolet (UV) light from the sun reacts with nitrogen oxides (NOx) and volatile organic compounds (VOCs). It is visible as a yellow-brown haze during the morning and in the afternoon. Photochemical smog tends to occur more often on dry summer days when the region experiences the most sunlight.
It is characteristic of urban areas and is thus commonly found in densely populated cities such as Los Angeles, Sydney, Mexico, New Delhi, and Beijing, among many others. It is also sometimes referred to as ‘Los Angeles Smog.’
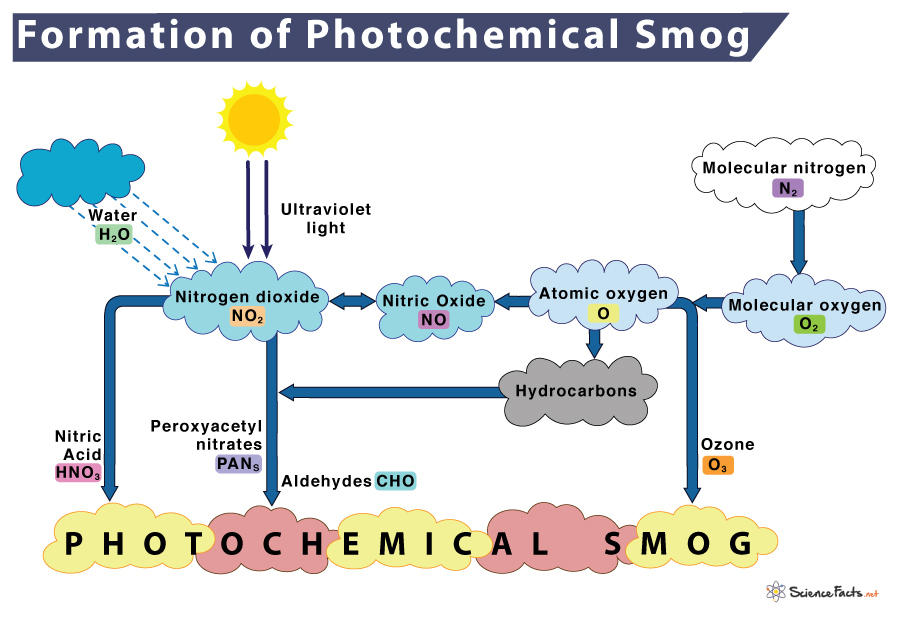
Photochemical smog requires neither smoke nor fog. It mainly consists of nitrogen dioxide (NO2), ozone (O3), peroxyacetyl nitrate (PAN), and organic compounds containing the aldehyde group.
This air pollution is formed when solar radiation reacts with primary airborne pollutants emitted from automobiles, industries, and power plants. Some common examples of primary pollutants are nitric oxide, nitrogen dioxide, nitrous oxide, and most VOCs.
During morning peak traffic hours, nitrogen oxides and VOCs are hugely released into the environment. Natural calamities such as volcanic eruptions, forest fires, and microbial activities also contribute to NO and NO2. When these primary pollutants are exposed to the sun’s ultraviolet radiation, NO2 goes through a series of complex reactions with hydrocarbons to form more secondary pollutants such as ozone, peroxyacetyl nitrate, nitric acid, aldehydes, and others. The combination of all these chemicals causes the formation of photochemical smog.
The stages for photochemical smog formation is given below along with the chemical equations that show how the components react with each other:
1) In the morning, nitrogen is burned or oxidized when released from automobiles to form nitric oxide in an oxidation step.
N2 (Nitrogen) + O2 (Oxygen) → 2NO (Nitric oxide)
2) Within a few hours, nitric oxide (NO) combines with more oxygen in another oxidation reaction to form nitrogen dioxide.
2NO + O2 (Oxygen) → 2NO2 (Nitrogen dioxide)
3) Nitrogen dioxide absorbs sunlight to breaks down, forming nitric oxide (NO) and oxygen radical (O) in a reduction reaction.
NO2 (Nitrogen dioxide) + Sunlight → NO (Nitric oxide) + O (Oxygen radical)
4) Oxygen radicals react to atmospheric oxygen (O2) to form ground-level ozone (O3).
O (Oxygen radical) + O2 (Atmospheric oxygen) → O3 (Ozone)
5) Ozone is consumed by nitric oxide to produce nitrogen dioxide and oxygen. This reaction is reversible.
O3 (Ozone) + NO (Nitric oxide) ⇌ NO2 (Nitrogen dioxide) + O2 (Oxygen)
6) Nitrogen dioxide reacts with volatile organic compounds (VOCs) such as hydrocarbons (R) to form secondary pollutants such as peroxyacetyl nitrate (PAN).
NO2 (Nitrogen dioxide) + VOCs (Hydrocarbons) → PAN (Peroxyacetyl nitrate) + Others
As discussed, photochemical smog is related to severe consequences in urban areas where automobile pollution is high. It is the responsibility of humanity to reduce its effect and to get the community involved. Some ways of controlling the formation of smog are as follows:
Ans. Photochemical smog typically occurs in dry and sunny areas. The emission from the burning of fossil fuels, including gasoline from vehicles and industry, reacts with other atmospheric organic chemicals in the presence of sunlight to form a thick haze. Photochemical smog is oxidizing in nature. In contrast, industrial smog is formed in cool and humid urban areas. It forms when factories burn fossil fuels creating smoke and sulfur dioxide that reacts with fog droplets to create a thick haze close to the ground-level. Industrial smog is reducing in nature.
Q2. From where does ozone come in photochemical smog?Ans. Ozone is a type of secondary pollutant formed when nitrogen oxides in the air react with volatile organic compounds in the presence of sunlight.
References